B
INFINI PREMIUM FILLER BODY
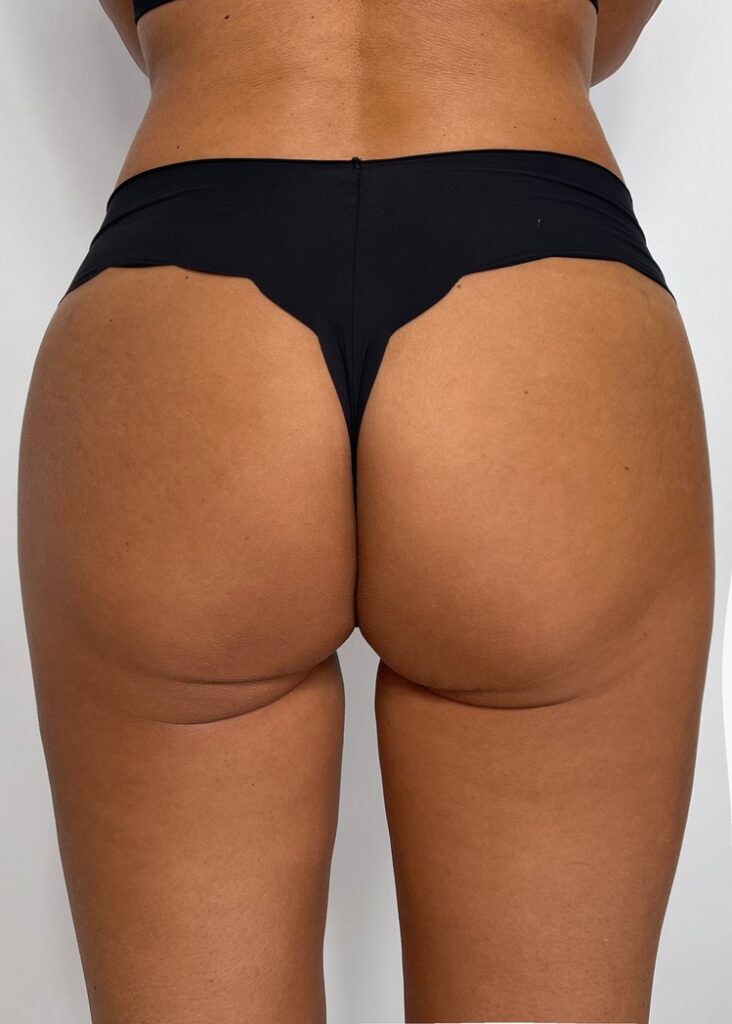
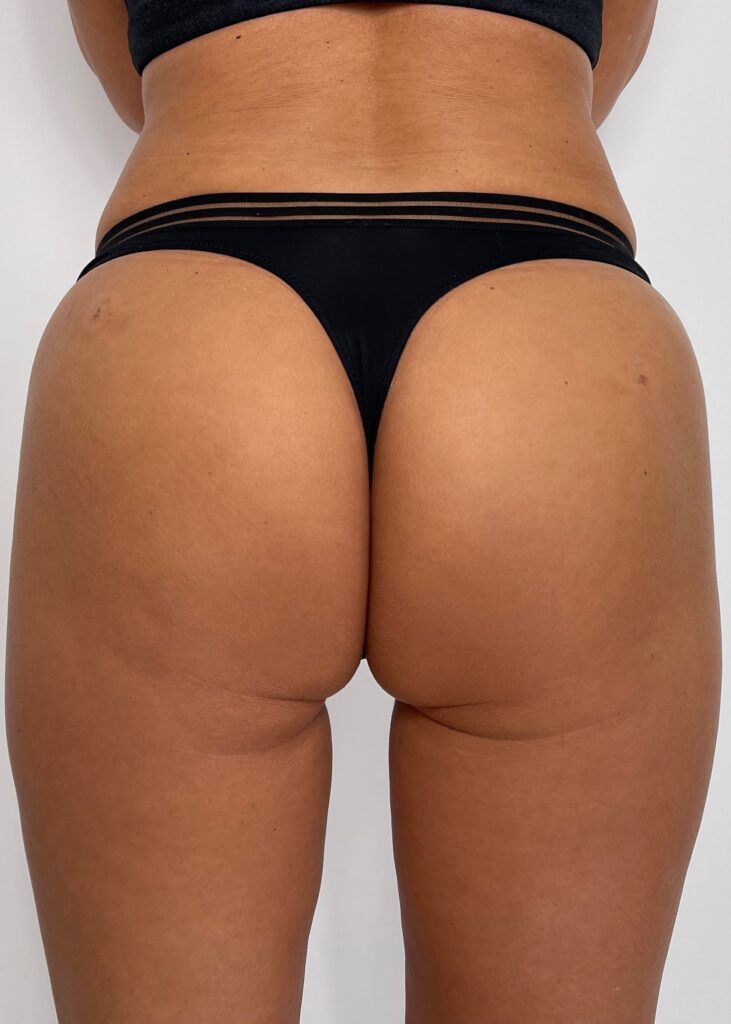
INFINI PREMIUM FILLER B BODY is a gel of crosslinked, highly concentrated hyaluronic acid dedicated to restoring or increasing the volume of soft tissues and modeling the body surface. It is perfect for adding youthful volume to the body as well as treating inequalities created after liposuction treatments and volumetric body treatments.
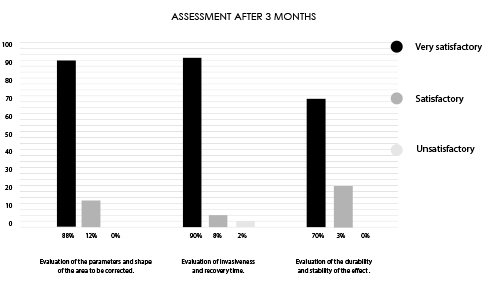
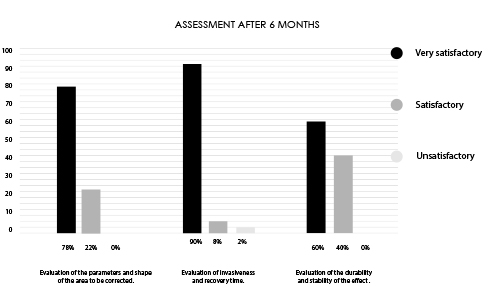
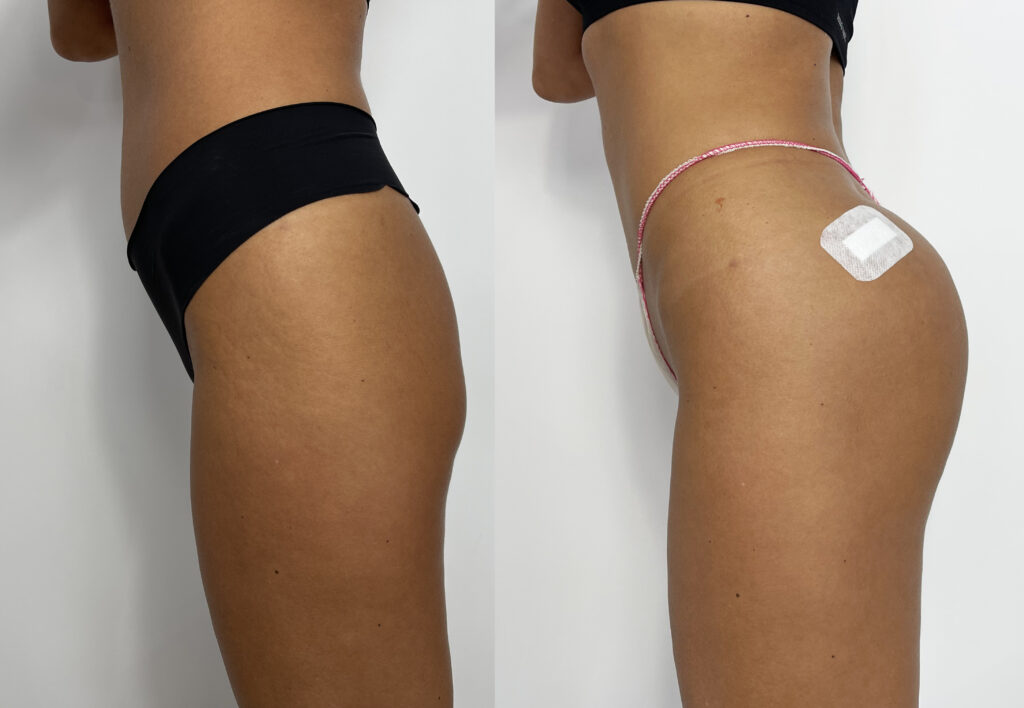
Designed for contouring the silhouette in areas such as buttocks, calves, or hands. INFINI Premium Filler B Body provides a visible volumetric correction by adding long lasting shape and volume to the tissue. INFINI Premium Filler Body unique properties allows practitioners to create enhancements to the tissue achieving client goals. Visibility and durability of the effect depends on the depth of application and the original condition of the skin. INFINI Premium Filler B Body injected into the skin increases the volume of tissue and fills the corrected skin defect.
Cross-linked hyaluronic acid 20mg/ml